Background
Asciminib is a first-in-class STAMP (Specifically Targeting the ABL Myristoyl Pocket) tyrosine kinase inhibitor (TKI). Its efficacy and safety profile in patients with chronic-phase chronic myeloid leukaemia (CML) have been shown in the Phase 3 ASCEMBL study, comparing asciminib 40 mg twice daily versus bosutinib 500 mg once daily. However, asciminib use in Chinese patients has hitherto not been reported. Moreover, the safety of high-dose asciminib (≥150 mg twice daily) in the Asian population is yet to be addressed. Here we present the real-world clinical outcomes of Chinese patients with Ph+ leukaemias in Hong Kong who received asciminib through a Managed Access Program (MAP).
Methods
Queen Mary Hospital (QMH) is a university-affiliated tertiary hospital in Hong Kong. Patients with Ph+ leukaemias under the care of QMH for whom asciminib was requested through MAP were included. Details on patient demographics, disease history, prior treatment lines, asciminib usage, adverse events, efficacy and death were collected.
Results
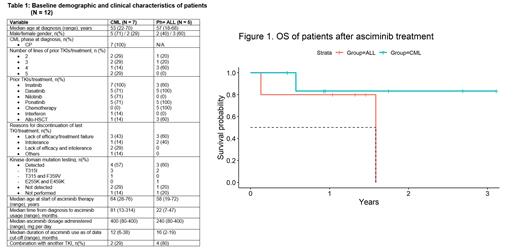
At data cut-off for analysis on 30 June 2023, 13 patients for whom asciminib was approved had data available. One patient passed away before the commencement of asciminib, and thus was excluded (Table 1). There were 7 patients with CML, who on diagnosis were all in chronic phase at a median age of 53 (22-70) years. All had received imatinib and at least one newer generation TKI, with 5 patients (71%) having received ponatinib. The majority (5 patients, 71%) received asciminib because of lack of efficacy of prior treatment, with 4 patients also having the T315I mutation. The median age at asciminib treatment (median dose: 400 mg, 80-400 mg) was 64 (28-76) years, with a median time from diagnosis of 81 (13-314) months. Two patients received imatinib concomitantly. The median duration of treatment was 12 (6-38) months. Two patients were already in at least major molecular response (MMR) before treatment, and have maintained that response. Two patients had ≥ 1-log reduction of BCR::ABL1 transcript level. Three patients had stable disease. There was one death unrelated to CML (Figure 1).
There were 5 patients with Ph-positive acute lymphoblastic leukaemia (ALL), at a median age of 57 (18-68) years at diagnosis. All had received prior chemotherapy in combination with a TKI (imatinib, N=3; dasatinib, N=5; ponatinib, N=5), with 3 patients having undergone allogeneic haematopoietic stem cell transplantation (allo-HSCT). Three patients had detectable kinase domain mutation (T315I, N=2; E255K + E459K, N=1). The majority (3 patients, 60%) received asciminib because of lack of efficacy of prior treatment. The median age at asciminib treatment (median dose: 240 mg, 80-400 mg) was 58 (19-72) years, with a median time from diagnosis of 22 (7-47) months. The median duration of treatment was 16 (2-19) months. Four patients received another ATP-competitive TKI concomitantly (imatinib, N=1; dasatinib, N=1; ponatinib, N=2). For patients receiving asciminib due to prior treatment failure (N=3), complete molecular response (CMR) was achieved in 2 patients with measurable residual BCR:: ABL1; 1 patient with frank leukaemia achieved complete remission with incomplete hematologic recovery with molecular response at 0.06%. For two patients receiving asciminib because of intolerance to prior treatment, one further improved from MR4 to CMR while pre-existing CMR was maintained in the other patient. There were 3 deaths; 2 due to COVID-19 and 1 due to refractory leukaemia (Figure 1).
Asciminib showed an acceptable safety profile, with raised lipase leading to dose reduction only in 1 patient. Grade 3 or above toxicities were all haematological, observed in 5 patients, 2 of whom had previous allo-HSCT. One patient on asciminib-ponatinib treatment developed grade 2 renal impairment.
Conclusion
Asciminib demonstrated promising clinical efficacy with satisfactory tolerance in this cohort of Ph+ leukaemias failing multiple lines of therapy. Further investigation of its therapeutic role in Ph+ ALL is warranted.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Use of asciminib in patients with advanced-phase chronic myeloid leukemia and Ph-positive acute lymphoblastic leukemia

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal